How Many Orbitals Are There in the Seventh Shell
Also know how many orbitals are in the 7th shell. View the full answer.
How Many Electrons Can The Third Energy Level Hold At Level
Click hereto get an answer to your question How many orbitals are there in the seventh shell.

. 100 2 ratings for n1 shell the first shell. In the seventh shell 49 orbitals are present so total electrons present in 49 orbital is 98. In the seventh shell 49 orbitals are present so total electrons present in 49 orbital is 98.
Of orbitals n2. The maximum electron an orbital can hold is two and both the electron should have the opposite. How many orbitals are there in the seventh shell.
The maximum electron an orbital can hold is two and both the electron should have the opposite. In the seventh shell 49 orbitals are present so total electrons present in 49 orbital is 98. For any atom there are three 7p orbitals.
Therefore the total number of orbitals present in the seventh shell is 49. For a multielectron atom a 3s orbital lies lower in energy than a 3p orbital because. A surface where there is no chance of finding the electron.
The fourth shell has 4 subshells. In 5th shell there would h orbital and this can be explained by the electronic configuration and number of electrons per shell 1st shell - 1s2. This gives a total of four orbitals.
For n1 there is only the 1s orbital. The maximum electron an orbital can hold is two and both the electron should have the opposite. More specifically the number of orbitals that can be found in each shell is given by.
1357 sum of first four odd nos U can simplify this result as total orbitals in nth shell is equal to sum of first n odd nos. For n2 there are three 2p orbitals in addition to the 2s orbital. In the n1 shell you only find s orbitals in the n2 shell you have s and p.
Therefore the total number of orbitals present in the seventh shell is 49. Of orbitals 22 4. For subshell d there are 5 orbitals.
There is one orbital in the 3s subshell and three orbitals in the 3p subshell. The s subshell which has 1 orbital with 2 electrons the p subshell which has 3 orbitals with 6 electrons the d subshell which has 5 orbitals with 10 electrons and the f subshell which has 7 orbitals with 14 electrons for a. Are there 7 orbitals.
Is there a 7 p orbital. There are nine orbitals in the n 3 shell. The maximum electron an orbital can hold is two and both the electron should have the opposite.
It has 1s orbital An s-orbital holds 2 electrons. How many electrons in the third shell. _____ Ne3s73P5 Kr4d12 5s2 5p1 Ar3d10 4s2 4p5 onlyA onlyB B and C A and B The absorption of a.
It depends on the shell. The maximum electron an orbital can hold is two and both the electron should have. 1 6 2 7 3 21 4 49.
This gives seven extra orbitals so for n 4 there are 9 7 16 orbitals. The first shell can contain at most 2 electrons the second and third shells 8 the fourth and fifth shells 18 the sixth and seventh shells 32 even if it is actually completed with 26 electrons table 1. Therefore the total number of orbitals present in the seventh shell is 49.
In reality there arent any atoms on the periodic table that have 50 electrons in their fifth shell nor any that have 72 electrons in their 6th shell or 98 electrons in their 7th shell etc Due to the peculiarities of quantum mechanics electrons start filling higher shells before the lower shells are complete. For subshell f there are 7 orbitals. Now u r wondering then how came n2how it can give the desired result u can observe that the total orbitals in the above ques are given by.
If there are multiple orbitals with the same energy the electrons tend to stay as far apart as possible. Therefore the total number of orbitals present in the seventh shell is 49. Therefore the total number of orbitals present in the seventh shell is 49.
So for the first shell you have. 2 Orbitals are combined when bonds form between atoms in a molecule. Each principal energy level above the first contains one s orbital and three p orbitals.
There are four types of orbitals that you should be familiar with s p d and f sharp principle diffuse and fundamental. Now add them up - u will get 16 orbitals. Many orbitals are there in the seventh shell n7.
One orbital can be in 7s. Correspondingly how many orbitals are in the 7th shell. How many orbitals can 7s have.
The number of orbitals in a given subshell such as the 5d subshell is determined by the number of possible values of which quantum number. _____ 6 7 49 21 A neutral P atom has many valence electrons. This gives seven extra orbitals so for n 4 there are 9 7 16 orbitals.
Therefore the total number of orbitals present in the seventh shell is 49. Of orbitals 12 1. Therefore n1 shell can hold two electrons.
Subsequently question is how many orbitals are in each energy level. For an orbital a node is. Within each shell of an atom there are some combinations of orbitals.
How many orbitals are there in the seventh shell. _____ 16 1 3 6 Which of the following represent electron configuration that violate the Pauli exclusion principle. Answer 1 of 4.
5s2 5p6 5d10 5f14 5h18. For the second shell you have. In the seventh shell 49 orbitals are present so total electrons present in 49 orbital is 98.
B 4 1 -1 -12 An electron in a 4p orbital can have which set of quantum numbers n l. For n3 there are five 3d orbitals three 3p orbitals and one 3s orbital giving a total of nine. In your case the seventh shell.
How many orbitals are in Level 7. 4s2 4p6 4d10 4f14. The n 3 shell however also includes 3d orbitals.
These orbitals have the same shape but are aligned differently in space. Other electrons more effectively shield electrons in the 3p orbital from the nucleus. Additionally how do you find the number of Subshells.
How many orbitals are there in the seventh shell.
How Many Orbitals Are In N 7 Quora

Orbitals Quantum Numbers Electron Configuration Multiple Choice Practice Problems Youtube
What Is The Maximum Number Of Electrons That Can Fit In Each Of The First Four Electron Shells Quora

How Many Electrons Can The Third Energy Level Hold At Level
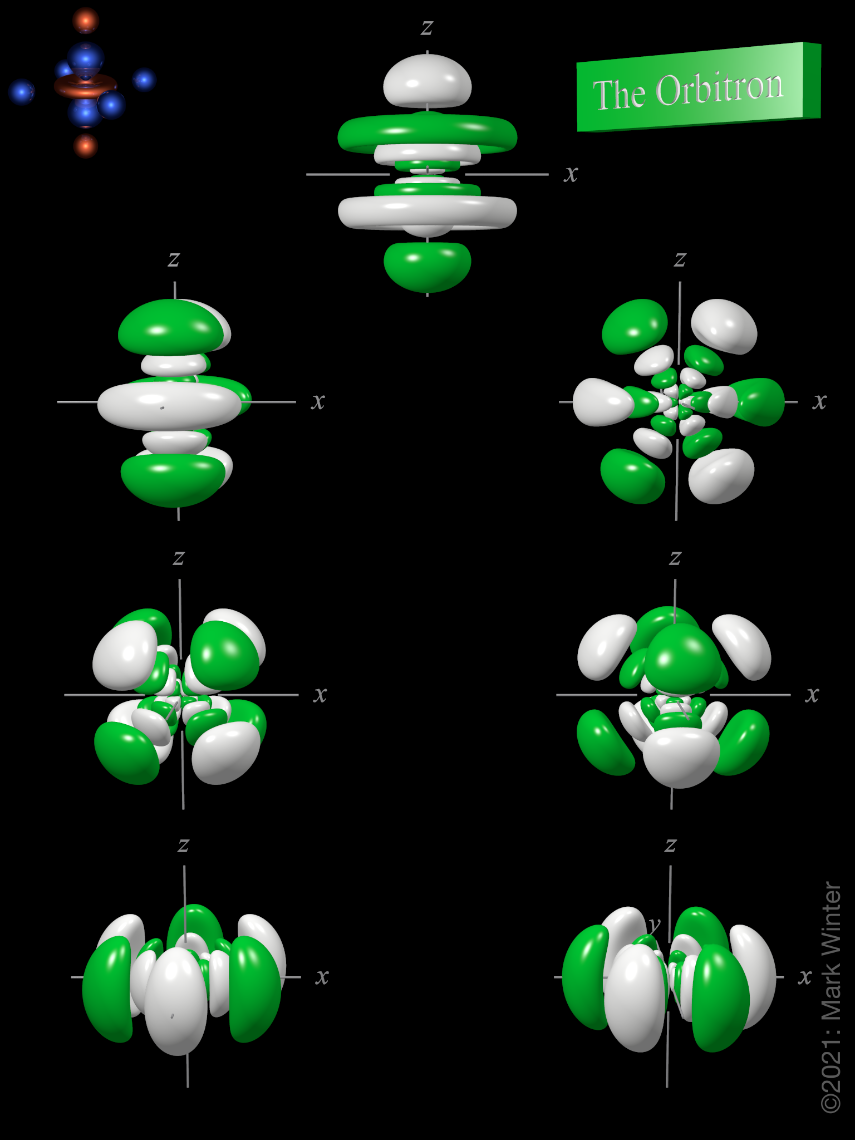
The Orbitron 7f Atomic Orbitals
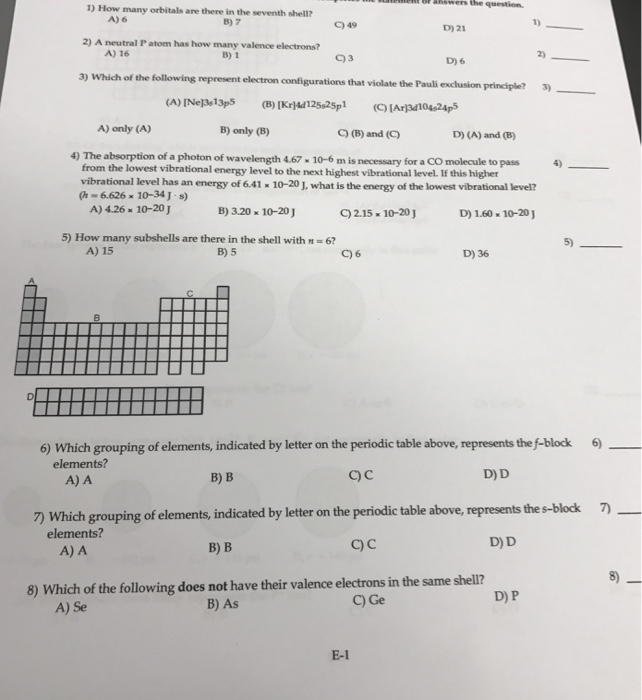
Solved How Many Orbitals Are There In The Seventh Shell Chegg Com
How Many Sublevels Are In The Third Energy Level At Level

Ionic Vs Covalent Coloring Activity Chemistry Science Pdf Printable From Laurelsusanstudio On Teacher Chemistry Lessons Teaching Chemistry Chemistry Classroom

How Many Orbitals Are There In N 3 The 3rd Energy Level Of An Atom Youtube

How Many Orbitals Are In N 7 Quora

The Bohr Model And Atomic Orbitals Using An Element S Position In The Periodic Table To Predict Its P Chemistry Lessons Teaching Chemistry Chemistry Classroom

How Many Orbitals Are In N 7 Quora

How Many Orbitals Are In N 7 Quora

Molecular Orbital Educational Infographics To Help Stem Students Chemistry Organic Chemistry Introduction To Organic Chemistry

How Many Orbitals Are In N 7 Quora

How Many Orbitals Are In N 7 Quora

Periodic Elements Electron Shells Subshells And Orbitals Chemistry


Comments
Post a Comment